Arsenic Removal
Arsenic is a common, naturally occurring drinking water contaminant that originates from arsenic-containing rocks and soil and is transported to natural waters through erosion and dissolution.
Arsenic occurs in natural waters in both organic and inorganic forms. However, inorganic arsenic is
predominant in natural waters and is the most likely form of arsenic to exist at concentrations that
cause regularity concern.
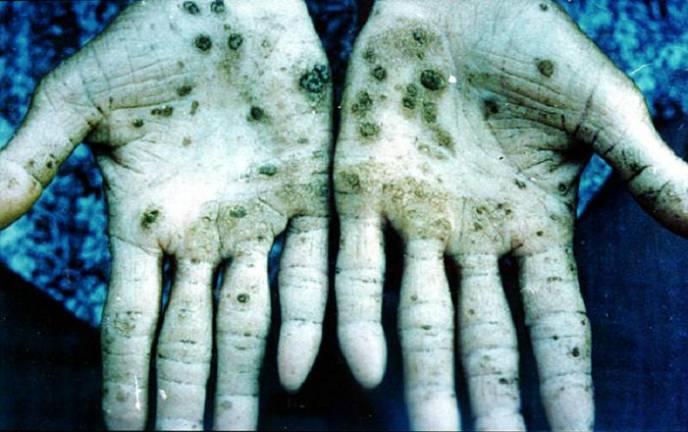
Excessive amounts of arsenic can cause acute gastrointestinal and cardiac damage. Chronic doses can cause vascular disorders such as blackfoot disease, and epidemiological studies have linked arsenic to skin and lung cancer.
A maximum contaminant level for arsenic is set at 0.05 mg/l.
Excessive amounts of arsenic can cause acute gastrointestinal and cardiac damage. Chronic doses can cause vascular disorders such as blackfoot disease, and epidemiological studies have linked arsenic to skin and lung cancer.
A maximum contaminant level for arsenic is set at 0.05 mg/l.

To remove arsenic from drinking water, water is passed through one or more IX resin beds. Arsenate
ions (H2AsO4
-
and HAsO4
2-) and several other anions (most notably sulfate) are preferentially removed
according to the order of preference for exchange. When all available sites on the resin have been
exhausted, the bed is regenerated with a brine solution (chloride exchange).
Ion exchange is a proven, well known technology that has been used commercially in a wide variety of applications for over 50 years. Ion exchange has been designated as a "Best Available Technology" by the EPA (USA) for the removal of arsenic from drinking water sources.
Ion exchange is a proven, well known technology that has been used commercially in a wide variety of applications for over 50 years. Ion exchange has been designated as a "Best Available Technology" by the EPA (USA) for the removal of arsenic from drinking water sources.
